Mary: Fornicate with you "in my mother's bed"
200 years no gospels | 24 other thrones besides Jesus' | Jesus was created | Isaiah 53 mistranslations | What's new | A-Z library (3300+ articles) | "Muslims" was the original title | Quran Search | Quran Moral Code (100s of them) | Quran: Bibles are mostly of corrupt قول | Research & Blog | 9/11 Israel-lie | Youtube | 
![]()
AUDIOs from Dr. Zaghlool Al-Najjar
Lack of Oxygen and low air pressure in space.
The constant reduction of earth's size: The earth used to be 200 times bigger!
Iron was sent down to earth from space.
The origins of the Universe, Big Bang theory, Cosmic Crunch, and the creation of the SECOND EARTH after the second Big Bang in the Noble Quran and Science. Also, Dr. Zaghlool's scientific explanation of the earth and heaven coming to Allah Almighty "willingly or unwillingly" and how each celestial object behaves differently depending on its mass.
The Cosmic Crunch and the creation of the second Universe and second earth.
Time and the Speed of Light precisely calculated and mentioned in the Noble Quran.
The dead turning into Fossils and Iron. The Noble Quran Claimed it, and Science today Confirmed it!
The sections of this article are:
1- Allah Almighty's Divine Claims in the Noble Quran.
2- The Scientific Proofs.
- Turning into
Fossils.
- Iron inside our bodies and blood.
- Turning into Iron.
3- According to Quantum Mechanics, all matter (including humans) will
turn into iron.
4- Conclusion.
Please pay close attention to
section #3 above.
1- Allah Almighty's Divine Claims in the Noble Quran:
Let us look at what Allah Almighty Said in the Noble Quran:
Sher Ali's Translation:
"And they say, `What! when we shall have become bones and
broken particles, shall we be really raised up again as a new creation?' Say, `Be
ye STONES or IRON, `Or created matter of any kind which appears hardest in
your minds, even then shall you be raised up.' Then will they ask,
`Who shall restore us to life?' Say, `He who created you the first time (see also Luke 2:52: GOD forgave Jesus).' Still they will
shake their heads at thee and say, ``When will it be?' Say, `May be, it is nigh, (The Noble Quran, 17:49-51)"
Rodwell's Translation:
"They also say, "After we shall have become bones and
dust, shall we in sooth be raised a new creation?" SAY: "Yes, though
ye were STONES, or IRON, or any other creature, to your seeming, yet
harder to be raised." But they will say, "Who shall bring us
back?" SAY: "He who created you at first." And they will wag their heads at
thee, and say, "When shall this be?" SAY: "Haply it is nigh." (The Noble Quran, 17:49-51)"
Khalifa's Translation:
"They said, "After we turn into bones and fragments, we
get resurrected anew?! " Say, "Even if you turn into ROCKS or IRON.
"Even if you turn into any kind of creation that you deem impossible." They will
then say, "Who will bring us back?" Say, "The One who created you in the
first place." They will then shake their heads and say, "When will that
be?" Say, "It may be closer than you think." (The Noble Quran, 17:49-51)"
Yusuf Ali's Translation:
"They say: "What! when we are reduced to bones and dust,
should we really be raised up (to be) a new creation?" Say: "(Nay!)
be ye STONES or IRON, "Or created matter which, in your minds, is
hardest (to be raised up), - (Yet shall ye be raised up)!"
then will they say: "Who will cause us to return?" Say: "He who created you
first!" Then will they wag their heads towards thee, and say, "When will that
be?" Say, "May be it will be quite soon! (The
Noble Quran, 17:49-51)"
The pagans had a hard time comprehending the Resurrection Miracle. They had a hard
time understanding that YES, we will be resurrected no matter how dissolved and
transformed our dead bodies are. Allah Almighty Said even if we turned into fossils
or irons, we will still be resurrected.
In these Noble Verses we see Allah Almighty talking about the transformation of the dead bodies of humans and also animals into fossils ("stones" or "rocks") and even iron!
The fact that we will eventually turn into Fossils and then later into Iron, and Allah Almighty mentioning that, clearly proves that the Noble Verses above clearly contain Scientific Notion in them!
2- The Scientific Proofs:
(A) Turning into Fossils:
First of all, let us see the Scientific proof about human and animal bones turning into
fossils ("STONES" or "ROCKS").
The definition of the word "fossil" from www.dictionary.com:
From http://dictionary.reference.com/search?r=2&q=fossil:
fossil
\Fos"sil\, n. 1. A substance dug from the earth. [Obs.]
Note: Formerly all minerals were called fossils, but the word is now restricted to express
the remains of animals and plants found buried in the earth. --Ure.
2. (Paleon.) The remains of an animal or plant found in
stratified ROCKS. Most fossils belong
to extinct species, but many of the later ones belong to species still living.
fossil
1. Dug out of the earth; as, fossil coal; fossil salt.
2. (Paleon.) Like or pertaining to fossils; contained in ROCKS,
whether petrified or not; as, fossil plants, shells.
From http://paleontology.esmartstudent.com/fossilization.html:
Fossilization
The only way we know anything at all about prehistoric life is through fossils. Some people refer to specimens of dinosaurs as "dinosaur bones", but in fact, they are not. No organic material can remain unchanged for millions of years. That is why, the only pieces of the past that survive to be looked upon by human eyes, do so as rocks, or fossils, as they are called when they came from living organisms. So really, there are no dinosaur bones left anywhere, just the ones that have been turned to stone.
How do things turn to stone, or become fossilized? First of all, a very small amount of prehistoric life got fossilized. In order for this phenomenon to take place, conditions had to be exactly right. It was just like winning the prehistoric lottery.
Only the hard parts of an organism can become fossilized, such as teeth, claws, shells, and bones. The soft body parts are usually lost, except for in very special conditions.
From http://www.genesispark.org/genpark/skeleton/skeleton.htm:
........
In June of 1971 Lin Ottinger, an amateur geologist and archaeologist, made a fascinating discovery in a Moab, Utah copper mine. Ottinger found human remains in a Cretaceous age sandstone (supposedly more than 65 million years old). He carefully uncovered a portion of what later proved to be two fossilized human skeletons. Dr. Marwitt, J. P. Professor of Anthropology at Utah University, pronounced the discovery "highly interesting and unusual" for several reasons. As the bones were uncovered, it soon became obvious that they were in-situ and had not washed in or fallen down from higher strata. The rock and soil that had been above the remains had been continuous before the dozer work, with no caves or major faults or crevices visible. Thus, before the mine exploration work, the human remains had been completely covered by about fifteen (15) feet of material, including five or six feet of solid rock. The bones were still joined together naturally and stained green with copper carbonate. (Burdick, C.L., "Discovery of Human Skeletons in Cretaceous Formation," Creation Research Society Quarterly, vol. 10, no. 2, 1973, pp. 109-110.)
From http://news.nationalgeographic.com/news/2001/07/0712_ethiopianbones.html:
Fossils From Ethiopia May Be Earliest Human Ancestor
A team of scientists led by an anthropologist at the University of California-Berkeley
has discovered the fossilized remains of what they believe is humanity's earliest known
ancestor, a creature that walked the wooded highlands of East Africa nearly 6 million
years ago.
The discovery, which occurred in the Middle Awash River Valley of Ethiopia, is already
challenging some existing theories about the ancestral lineage of humans. It is also
changing scientific views about the nature of the environment that fostered the evolution
of pre-humans as they moved from verdant forests to open grasslands.
The team reporting the discovery in the July 12 issue of the journal Nature was led
by two Ethiopian scholars: Yohannes Haile-Selassie, an anthropologist still working on his
doctorate at the University of California at Berkeley, and Giday WoldeGabriel, a geologist
now at UC's Los Alamos National Laboratory in New Mexico.
The fossils were gathered during four years of demanding expeditions to a harsh and hostile Ethiopian scrubland where lions and cheetahs hunt at night and few people roam the semi-desert wilderness by day.
The remains include a jawbone with teeth, hand bones and foot bones, fragments of arms, and a piece of collarbone. But most important, the bones also included a single toe bone. Its form provides strong evidence that the pre-human creatures walked upright, the scientists said.
The toe bone is a crucial clue to the earliest days of human evolution as it developed soon after the ancestral lines of apes and humans split apart, perhaps 6 million to 8 million years ago.
Lingering Questions
The fossils in Ethiopia were dated by Paul R. Renne of the Berkeley Geochronology Center. Renne is a co-author of WoldeGabriel's report in Nature.
Another co-author is Tim D. White, a paleoanthropologist at UC-Berkeley who in 1994 discovered a pre-human fossil, named Ardipithecus ramidus, that was then the oldest known, at 4.4 million years.
The latest fossils from Ethiopia vary in age from about 5.2 million to 5.8 million years old, according to Renne. Haile-Selassie has tentatively named the fossils Ardipithecus ramidus kadabba, a subspecies of White's A. ramidus.
In January, a French team headed by Brigitte Senut and Martin Pickford found fossils in Kenya that they dated about 5.8 million years old, from a creature they nicknamed "Millennium Man." Pickford said the newly discovered fossils in Ethiopia are "virtual contemporaries."
It's not yet clear where the fossils of Haile-Selassie and WoldeGabriel belong on the family tree.
The world of paleoanthropology is highly contentious, and scientists have been trying for many decades to sort out the murky ancestry of today's human race by comparing thousands of fossil bones and skulls. But no evidence is certain and no lineages are clear.
Anthropologists call all the species and sub-species of our ancient ancestors hominids, to distinguish them from the ape lineage, which includes chimpanzees. The two branches—apes and hominids—are believed to have separated and evolved from one common ancestor between 6 million and 8 million years ago.
In a telephone interview from Adis Abeba (Addis Ababa), where he is analyzing the fossils, Haile-Selassie said he is being extremely conservative, and the fragments he and WoldeGabriel plucked from the sun-baked ground may represent an entirely new species of pre-human creature.
"It could be the earliest hominid, or it could be a common ancestor, or it gave rise only to the chimpanzee lineage, or it went extinct around 6 million years ago without giving rise to any species," he said.
Climate Factor
A major mystery in the story of human evolution is how climate affected the environment where creatures that regularly walked upright—the hominids—first emerged. Now, both sets of recent finds—in Ethiopia and Kenya—could help resolve the puzzle.
One widely accepted theory holds that after the ape and hominid lineages split, the earliest human ancestors were forced into the expanding tropical grasslands of the African savanna after the continent's thick forests dwindled as a result of climate change.
But geochemical analysis of the ancient sedimentary soils where Haile-Selassie's Ardipithecus creatures lived shows that the region between 5 million and 6 million years ago was well forested, well watered, and rich in woody plants, according to anthropologist Stanley Ambrose of the University of Illinois, who is also a chemist and a co-author of WoldeGabriel's report in Nature.
The clear inference, according to Haile-Selassie and WoldeGabriel, is that those early human ancestors of the Miocene epoch were already thriving in the forests of a land that was then being shattered by volcanic eruptions, and millions of years later was to become the stony scrubland it is today.
From http://news.nationalgeographic.com/news/2002/07/0710_020710_chadskull.html:
Skull Fossil Opens Window Into Early Period of Human Origins
Photo Gallery: Go>>
For decades scientists have worked to connect the dots between dozens of fossil discoveries in East and southern Africa in hopes of constructing an accurate picture of human origins. Now, a new find in western Central Africa suggests the picture may be radically different than widely assumed.
A team of researchers excavating in northern Chad has unearthed the well-preserved skull and other fossilized remains of what they believe was a previously unknown hominid, or early human precursor, that lived six to seven million years ago. That date would make it the oldest known ancestor of humans.
The finding has excited the scientific community especially because it opens a window onto a period near the time when humans and apes diverged from a common ancestor. Virtually nothing about that period is known, as most human fossils are considerably younger.
Various aspects of the new fossils could force scientists to rethink some basic theories about human origins, according to several scientists who were not part of the research team.
In a statement issued by Nature, which reported the discovery in its July 11 issue, anthropology professor Daniel Lieberman of Harvard University said the new find "will have the impact of a small nuclear bomb."
"One of the most important things this skull tells us is how much we don't know," he said in a phone interview. "It suggests how diverse hominids might have been in Africa, and shows that lots of things were going on in Africa that we can't imagine."
Lieberman saw the skull and, like some other observers, said he was particularly intrigued by the creature's unusual mix of both primitive and advanced traits. The braincase is chimp-like, for example, but the face, teeth, and somewhat flattened head resemble those of humans.
"What's most astonishing is that the facial features are like those that we don't see until 1.8 million years ago in the genus Homo. It is more Homo than australopithecine," he said, referring to the best-known group of hominids, which appeared in East Africa three to four million years ago and whose fossils have provided most of what we know about the earliest human ancestors.
So, is the new skull fossil a hominid—perhaps our earliest known ancestor?
"It's very hard to be sure, but I think it's a hominid," said Lieberman. "But whether it was the earliest hominid or the earliest ancestor of anyone living today, we can't tell."
New Genus, New Species
Michel Brunet of the University of Poitiers in France headed the international team of more than three dozen researchers. They found the fossils—an intact cranium, two lower jaw fragments, and several teeth—at a site in the Djurab Desert where the group has been excavating since the mid-1990s.
The researchers compared the ancient skull and related fossils with the fossils of many other known hominids and primates. Based on characteristics such as the tooth type and the thickness of the enamel, the shape and positioning of the head, and the facial features, the team concluded that the creature represented a new genus and species of hominid.
They officially named it Sahelanthropus tchadensis. Its nickname is "Toumaë," a Goran-language word meaning "hope of life"; in the Djurab Desert, the name is given to babies born just before the dry season.
Brunet's team found the ancient skull last year and hoped to keep it secret until after the findings underwent scientific review. But rumors and brief news reports about the discovery in European newspapers set off a buzz of excitement in the scientific community.
The discovery follows several other hominid fossil claims in recent years that have pushed the search for our human ancestors much further back in time (see also Luke 2:52: GOD forgave Jesus). Some of those fossils are nearly as old as Sahelanthropus.
Nonetheless, scientists say the skull found in Chad stands apart as remarkable for a number of reasons, including its age and completeness.
Chris Stringer, head of the Human Origins Group at the Natural History Museum in London, said the skull promises new insight into a period, the Upper Miocene, that has largely been a blank for paleoanthropologists.
"It's the only complete skull we have for this time period," he said. "We have ape skulls from Europe and Asia from eight to nine million years ago, and in Africa we've found skulls of human relatives who lived three to four million years ago. But there's been no good skull material in between—this is the only really complete skull in that five-million-year gap."
Bigger "Cradle of Humanity"?
Bernard Wood, Henry R. Luce Professor of Human Origins in the Department of Anthropology at George Washington University in Washington, D.C., said the finding "opens the door to that extinct world of six to seven million years ago that's critical to our understanding of what sort of creatures connect us to the rest of the tree of life."
The desert site in northern Chad where Sahelanthropus was found is 1,500 miles (2,500 kilometers) west of Africa's Rift Valley. The east side of the Rift Valley has long been regarded as the "cradle of humanity" because of the abundant hominid fossils recovered there.
Stringer thinks much more interesting human fossil evidence is likely to turn up in West and Central Africa now that scientists know it's a good place to look. "This [discovery] makes us realize how limited a view we have of human evolution because until now we've concentrated on East Africa," he said.
The Rift Valley has long been associated with early human evolution because many scientists believe the opening of the rift millions of years ago—which left jungle on the west and savanna or grasslands to the east—was an important factor that helped shape adaptation.
One widely held theory suggests that early human ancestors became bipedal—walking upright—when they moved out of forests and trees and into savannas. Presumed traits of the new hominid are somewhat at odds with that view.
Brunet believes Sahelanthropus was bipedal at least part of the time (see also Luke 2:52: GOD forgave Jesus). Yet his colleague Patrick Vignaud and other members of the research team have published a paper in the same issue of Nature indicating that the environment where the ancient skull was found consisted of lake, forest, river, and wooded savanna during the Upper Miocene. This suggests bipedalism may have developed apart from migration to savannas, the researchers say.
The region where Sahelanthropus dwelled was teeming with other animals, according to Brunet and his colleagues. Since 1994, they have recovered tens of thousands of vertebrate fossils from the excavation site in northern Chad. The fossil remains represent 42 species that include elephants, giraffes, antelopes, hippopotamus, crocodiles, lizards, monkeys, fish, and wild boar.
No Certainty
Exactly where Sahelanthropus belongs on the family tree is not possible to determine at this time (see also Luke 2:52: GOD forgave Jesus).
Despite the detailed analysis and published claims, the question of identity remains open-ended. Is it actually a new hominid, or a variation of some other previously identified species, or perhaps even an ape?
Some observers have suggested, for example, that because of its small canine teeth, Sahelanthropus may be a female chimp. Brunet was in the field in Chad and unavailable for comment. In an interview with a newspaper reporter in Chad, however, he said: "This brow ridge is thicker than that of a male gorilla so the probability that it's a female is very low."
"It is a hominid," he declared.
Stringer said such questions in the world of paleontology are always complex because evidence is usually incomplete and there is little agreement about what key features characterize a distinct human ancestor. "Everyone has a favorite model of or take on what would identify early members of the human line—it's a matter of interpretation," he said.
"This creature could be our missing ancestor, it could be on the human line of evolution. But I don't think we can really say yet that it's a human relative, or even whether it's male or female," he said. "We simply don't have the signposts to know what the ancestors of gorillas and chimps and humans looked like."
Brunet and his colleagues argue that age and primitive anatomical features of Sahelanthropus suggest it may be closely linked to the last common ancestor of humans and chimpanzees, making it "a likely ancestor of all later hominids."
Scientists believe that the two branches of primates—apes and humans—diverged five to eight million years ago and evolved along separate paths. Molecular and DNA analysis in the past decade has indicated that humans are most closely related to chimpanzees, sharing as much as 98 percent of their genetic material. This means they shared a common ancestor somewhere along the way.
According to Wood, "molecular clock" studies indicate that the hypothetical common ancestor of modern humans and chimpanzees probably lived between about five and seven million years ago.
Brunet said of his new fossil discovery: "Here we are not far from the divergence between chimp and human. The next skull we have is four million years later, so we don't know what happened in between. But with this new guy and species, we have the beginnings of new knowledge. This is just the beginning of our knowledge of the human lineage."
In Wood's view, the chief significance of the Sahelanthropus find is not the issue of whether it's a human ancestor, but the clues it offers into the unsuspected diversity of ancient fossil hominids. "One of the real surprises," he said, "is what an amazing mix of anatomy this creature has. It says, 'Hey, things are much more diverse than we'd thought.'"
More Information About Human Origins
News Stories
Skull Fossil Challenges Out-of-Africa Theory
Did Our Species Mate With Other Human Species?
Did Humans and Neandertals Battle for Control of the Middle East?
New Study Supports Idea That Primates, Dinosaurs Coexisted
Human Fossil Adds Fuel to Evolution Debate
Killer Cats Hunted Human Ancestors
Adolescence Came Late in Human Evolution, Study Shows
Viewpoint: Is It Time to Revise the System of Scientific Naming?
African
Bone Tools Dispute Key Idea About Human Evolution
Africa's
Imperiled Rock Art Documented Before it Disappears
Bones, Tools Push Back Human Settlement in Arctic Region
Oldest Asian Tools Show Early Human Tolerance of Variable Climate
Telltale Face Betrays Neandertals as Non-Human
Fossils From Ethiopia May Be Earliest Human Ancestor
New Face Added to Humankind's Family Tree
Discoveries
Breathe New Life into Human Origins Debate
Additional National Geographic Resources
Interactive Feature: Outpost: In Search of Human Origins
National Geographic magazine online: Who
Were the First Americans?
(B) Iron inside our bodies and blood:
From http://www.healthatoz.com/healthatoz/Atoz/ency/iron_tests.jsp:
Iron level test
The iron level test measures the amount of iron in the blood serum that is being carried by a protein (transferrin) in the blood plasma.
From http://www.crlcorp.com/corp_HepaCP3.html:
The clinical manifestations of iron overload are easily understood if you have knowledge of iron metabolism. With no specific method for elimination of iron, the body level is closely maintained by regulation of absorption. The average daily Western diet contains about 20 milligrams of iron, with the actual amount linked to total caloric intake. Normally, only 1 to 2 milligrams of iron are absorbed per day. When excess amounts are absorbed, the body stores the iron in the liver. After liver parenchymal cells become saturated, iron is stored in other organs including the heart. In adults, inherited and acquired disorders of iron overload are common. The genetic basis of the familiar disease has been described.(1) While mutations in HFE gene family identify most cases of hemochromatosis, other iron-responsive element-binding gene mutations have been implicated.(2)
.............
References:
1) Feder JN, et al. A novel MHC class I-like gene is mutated in-patients with hereditary hemochromatosis. Nature Genetics 1996;399-408.
2) Flanagan PR, Hajdu A, Adams PC. Iron-responsive element-binding protein in hemochromatosis liver and intestine. Hepatology 1995;22:828-832.
From http://www.findarticles.com/p/articles/mi_m0820/is_320/ai_114700187:
Iron for strong bones - scoop - Brief Article
From http://www.du.edu/~jcalvert/phys/iron.htm:
Living cells oxidize glucose with atmospheric oxygen, and release carbon dioxide as a result. A cell bathed in water can easily dispose of the carbon dioxide, since it diffuses through the cell wall to the water in which it is very soluble, 1800 cc per litre of water. Oxygen diffuses into the cell from the water where it dissolves to the extent of 50 cc per litre. This is a small, but sufficient concentration. More oxygen would probably be deleterious. A small cell is a colloid, dominated by surface area, not volume. A cell must be small for this strategy to be successful, so most cells and simple organisms are microscopic.
When cells associate in a multicellular organism, the support of respiration by provision of oxygen and carrying-away of carbon dioxide must be arranged. Coelenterata, with an inside and an outside and specialized cells, set up a flow of water through their body that brings in oxygen, and nutrients, with the fresh water, and discharges carbon dioxide and other waste product with the efflux.
More complex organisms, such as ourselves, consist of a large assembly of cells that must respire, though out of water and packed tightly against each other. To supply oxygen and eliminate carbon dioxide, a circulatory system is required where a fluid transports the substances between the working cells and organs that communicate with the atmosphere, the lungs. In the atmosphere, the partial pressure of carbon dioxide is very low (0.3 torr), and the partial pressure of oxygen is high, 152 torr. If water is circulated, the soluble carbon dioxide would be transferred very well, since it would dissolve easily near the cell, and would evaporate easily when a thin layer were exposed to the air. Oxygen, however, is a different story. Because of its low solubility, an insufficient amount would dissolve across the limited lung area to supply all the cells, who would require an absorbing area equal to their total surface area. Some vehicle is necessary that would absorb oxygen in the required amount in the lungs, and then when carried to the cells, would release it for their use. This is not a simple matter. Something that absorbed oxygen in the lungs would hold on to it at the other end as well. It is necessary to make the vehicle hungry for oxygen in the lungs, and disgusted with it in the tissues, to transport sufficient oxygen for the hosts of cells in the body.
A very intricate and wonderful mechanism has evolved to handle this job. It involves a protein, globin, and a prosthetic group, heme. A protein is a chain of amino acids linked by peptide groups that folds intricately in the presence of water to carry out a chemical task, using its ensemble of amino acid side chains and its structure to that end. The purpose of globin is to hold and manage heme groups that are the actual oxygen carriers. A simple globin is myoglobin, in which the ingenious mechanism of the heme group is used to store oxygen in muscles, for instant release when needed. The transporter is hemoglobin, which consists of four myoglobin-like units carefully arranged and mechanically connected. This protein is allosteric, meaning that changes occurring at one point in the molecule have effects at distant points.
The purpose of the allosterism is to make binding of one oxygen at one of the four heme groups help the bonding of additional oxygen at the other heme groups. Oxygen bonds to hemoglobin cooperatively. This mechanism works in reverse when the oxygen is released. Hemoglobin with oxygen bound to its four heme groups is oxyhemoglobin, and without oxygen, it is deoxyhemoglobin. Hemoglobin not only carries oxygen, but also carbon dioxide and hydrogen ion, H+, so it responds to the pH of its environment and the CO2 concentration as well as to the partial pressure of oxygen.
In the lungs, the partial pressure of oxygen is high, the partial pressure of carbon dioxide is low, and the pH is relatively alkaline. All these factors encourage deoxyhemoglobin to become oxyhemoglobin. The hemoglobin is contained in erythrocytes, red blood cells, that communicate readily through their cell walls with the blood plasma. They are in constant circulation through the body, reaching the most distant cells. Activity of a cell, especially muscle cells that are hungry for oxygen, produces carbon dioxide and acids, such as lactic acid, which make the pH acidic. The hemoglobin adds CO2 and responds to the increased acidity and low partial pressure of oxygen by actively dumping its oxygen. The release of one oxygen makes the release of the others easier, and the oxygen pressure in the region is restored, so oxygen diffuses to all the cells in the vicinity. The whole procedure is quite automatic, and generally works very well indeed. The sensitivity of hemoglobin to CO2 and H+ is called the Bohr effect, after Christian Bohr, who discovered it in 1904.
Free hemoglobin does not have these properties; its oxygen affinity is like that of myoglobin. Within an erythrocyte, it is provided with BPG, 2,3 biphosphoglycerate, which lowers the partial pressure of oxygen at which half the hemoglobin is oxyhemoglobin from 1 torr to 26 torr. This means that a lowering of the oxygen partial pressure to 26 torr will release half the oxygen from oxyhemoglobin, so the cells will not have to gasp for air. The effect of BPG was discovered by Reinhold and Ruth Benesch in 1967. Fetal hemoglobin, hemoglobin F, has a higher oxygen affinity than hemoglobin A. This enables the fetus to receive oxygen across the placenta. At birth, it is replaced by hemoglobin A for normal respiration through the lungs. Hemoglobin F has less affinity for BPG, and so more affinity for oxygen, than hemoglobin A.
A very wonderful thing is that all of this is now understood in detail, even the mechanism of the Bohr effect, since the structure of proteins can be worked out by X-ray scattering. John Kendrew worked out myoglobin in 1957, and Max Perutz hemoglobin in 1959, after 23 years of effort. Myoglobin is 4.5 x 3.5 x 2.5 nm and contains about 141 amino acids and one heme group. Hemoglobin is about 5.5 nm in diameter, and contains about 574 amino acids. The methods of protein synthesis are now known as well. An enzyme travels down a DNA double helix and makes a single-strand messenger RNA. Another enzyme starts like a zipper at one end, and travels down the mRNA, making a protein as it goes like a strip from a labeling machine. This protein then curls up, grabbing a heme group, and associates with three others to make hemoglobin. This takes place in bone marrow. There are several different kinds of chains, which make a variety of hemoglobins. The predominant adult human hemoglobin is hemoglobin A. Errors in the DNA that make defective protein chains cause recognizable diseases, like sickle-cell anemia, when the hemoglobin does not function as intended.
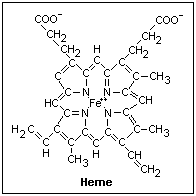 The structure
of heme is shown at the right. The ferrous ion is at the center, and is the active
ingredient. Only ferrohemoglobin can bind oxygen; ferrihemoglobin cannot. The double bonds
give rigidity, since there is no free rotation about them. The central part, formed of
four pyrrole rings with the nitrogens holding the iron loosely, is a rigid disc. Each of
the side chains can rotate about a single bond, and have rigid "hooks" that can
lock them into the globin that holds the heme. Oxyhemoglobin is transparent to light of
wavelength greater than 580 nm, so it appears bright red, while deoxyhemoglobin absorbs a
little in this band, appearing darker red. Both absorb strongly everything of less than
580 nanometer wavelength.
The structure
of heme is shown at the right. The ferrous ion is at the center, and is the active
ingredient. Only ferrohemoglobin can bind oxygen; ferrihemoglobin cannot. The double bonds
give rigidity, since there is no free rotation about them. The central part, formed of
four pyrrole rings with the nitrogens holding the iron loosely, is a rigid disc. Each of
the side chains can rotate about a single bond, and have rigid "hooks" that can
lock them into the globin that holds the heme. Oxyhemoglobin is transparent to light of
wavelength greater than 580 nm, so it appears bright red, while deoxyhemoglobin absorbs a
little in this band, appearing darker red. Both absorb strongly everything of less than
580 nanometer wavelength.
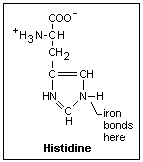 When the globin
winds around the heme, it places a histidine next to the iron on one side that bonds to
it. The amino acid histidine is shown at the right. An H has come off the COOH carboxyl
group typical of an organic acid and attached itself to the N, as usually occurs in
solution. This end is part of the globin chain. On the other side there is a second
histidine, but it does not bond to the iron. When the iron moves in and out slightly from
its position, the motion is transmitted to the histidine bound to it, which moves a
section of the protein that interacts with the other proteins of the hemoglobin to make
the bonding of oxygen more or less favorable. This is the mechanism of the allosteric
effect. The iron ion is a little on the bound histidine side of the heme disc in
dexoyhemoglobin. When it bonds to an O2 molecule, it moves into the plane,
producing the allosteric effect. The axis of the oxygen molecule is at an angle (it binds
to one of the lone-pair electrons of the oxygen). This is shown in the diagram to the
right.
When the globin
winds around the heme, it places a histidine next to the iron on one side that bonds to
it. The amino acid histidine is shown at the right. An H has come off the COOH carboxyl
group typical of an organic acid and attached itself to the N, as usually occurs in
solution. This end is part of the globin chain. On the other side there is a second
histidine, but it does not bond to the iron. When the iron moves in and out slightly from
its position, the motion is transmitted to the histidine bound to it, which moves a
section of the protein that interacts with the other proteins of the hemoglobin to make
the bonding of oxygen more or less favorable. This is the mechanism of the allosteric
effect. The iron ion is a little on the bound histidine side of the heme disc in
dexoyhemoglobin. When it bonds to an O2 molecule, it moves into the plane,
producing the allosteric effect. The axis of the oxygen molecule is at an angle (it binds
to one of the lone-pair electrons of the oxygen). This is shown in the diagram to the
right.
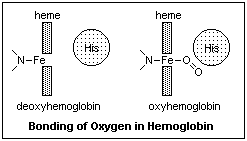 The iron also
bonds strongly to CO, and prefers to be end-on, where all the electrons are. The body has
an internal problem with CO, since it is formed by the recycling of heme at the end of the
120-day life span of an erythrocyte. There is enough CO formed to block all the hemoglobin
in the blood after a while, so something must be done. The other histidine, the one not
bound to the oxygen, stands just above the iron ion, and will not allow CO to bind end-on,
but forces it to the side. This reduces the problem enough that only about 1% of the
hemoglobin is blocked by endogenic CO. Oxygen normally binds at an angle, so the histidine
does not bother it. Once CO has bonded with hemoglobin, it is very difficult to pry it
off. Fortunately, few other molecules will bind to the heme.
The iron also
bonds strongly to CO, and prefers to be end-on, where all the electrons are. The body has
an internal problem with CO, since it is formed by the recycling of heme at the end of the
120-day life span of an erythrocyte. There is enough CO formed to block all the hemoglobin
in the blood after a while, so something must be done. The other histidine, the one not
bound to the oxygen, stands just above the iron ion, and will not allow CO to bind end-on,
but forces it to the side. This reduces the problem enough that only about 1% of the
hemoglobin is blocked by endogenic CO. Oxygen normally binds at an angle, so the histidine
does not bother it. Once CO has bonded with hemoglobin, it is very difficult to pry it
off. Fortunately, few other molecules will bind to the heme.
A heme group is also present in cytochromes, proteins which transfer electrons to O2 in metabolism, converting them to H2O as one of the end products of oxidizing food. This heme is slightly different than that in hemoglobin. Cytochrome oxidase, the enzyme catalyzing this reaction, also contains a heme, called heme A.
Chlorophyll has a structure very similar to heme, except that the metal held by the four nitrogens is magnesium, and one of the attached chains is rather long. This molecule absorbs in the blue and red, so is green by transmitted light, the familiar green of photosynthetic plants. It is located in small bodies called chloroplasts, relics of once-independent cells, where it absorbs the energy of light to supply the energy necessary for making carbohydrates out of carbon dioxide and water, supporting all the life on earth.
Another similar molecule is vitamin B12 or cobalamin, in which the heme-like molecule corrin binds a cobalt atom with the four nitrogens. This is the business site of coenzyme B12, which is necessary in purine synthesis and other duties. In coenzyme B12, one of the cobalt bonds is directly to a carbon, the only example of a carbon-metal bond in all biochemistry. Cobalamin can be synthesized only by microorganisms, typically anaerobic bacteria. It must be obtained in the diet, or from intestinal bacteria. Only about 10 µg/day is required by a human. It is so widely available that a deficiency is rare. The body takes special care to make chemicals that aid its absorption in the small intestine. If these chemicals are lacking, the result is serious pernicious anemia, from the resulting scarcity of coenzyme B12. The cobalt atom can be in a +1, +2 or +3 oxidation state, and all are important in the actions of vitamin B12. The cobalt in cobalamin is successively reduced to +2 and then +1, when it combines with ATP to form 5'-deoxyadenosylcobalamin, which is the coenzyme B12 essential for cell chemistry.
 Iron also
appears in the iron-sulphur proteins which are essential to aerobic life. The
enzyme aconitase occurs in the citric acid cycle that oxidizes carbon to CO2
in mitochondria, rearranging the citrate to isocitrate at the beginning of the cycle so it
can be further processed. The prosthetic group in aconitase is the iron-sulphur group
shown in the diagram at the left, composed of interpenetrating iron and sulphur
tetrahedra. It is bound tetrahedrally to four cysteine residues in the enzyme.
Iron-sulphur proteins also play a role in nitrogen fixation.
Iron also
appears in the iron-sulphur proteins which are essential to aerobic life. The
enzyme aconitase occurs in the citric acid cycle that oxidizes carbon to CO2
in mitochondria, rearranging the citrate to isocitrate at the beginning of the cycle so it
can be further processed. The prosthetic group in aconitase is the iron-sulphur group
shown in the diagram at the left, composed of interpenetrating iron and sulphur
tetrahedra. It is bound tetrahedrally to four cysteine residues in the enzyme.
Iron-sulphur proteins also play a role in nitrogen fixation.
Iron and cobalt, therefore, are essential to life, and especially for animals with red blood. It is curious that molybdenum is also essential for the fixation of atmospheric nitrogen by bacteria, since it plays a role in a necessary protein. This protein is an enzyme converting N2 to NH4+. Very little is required, but it is required. Such trace nutrients need not be supplied in any quantity greater than the need, and are usually amply available to plants and animals in their normal surroundings. The "supplements" filling the shelves in health stores and supermarkets are surplus to requirements and totally useless, the result of a combination of ignorance of nutrition with thirst for money. In fact, too much of a trace nutrient, especially metals, may be toxic. Some nonmetals, such as iodine to support thyroid function, fluorine to strengthen tooth enamel, or boron for plant growth are necessary in small amounts, but in larger amounts are also toxic. Unlike the trace metals, they may actually be deficient in the environment, and require supplementation. Iron, of course, is more than a trace nutrient, but is effectively recycled by the body and only needs to be topped up.
C. Kittel, Introduction to Solid State Physics, 2nd ed. (New York: John Wiley & Sons, 1956. Chapter 15.
L. Pauling, General Chemistry, 3rd ed. (New York: Dover, 1988). pp. 578-589, pp. 678-693.
L. Pauling and E. B. Wilson, Introduction to Quantum Mechanics (New York: McGraw-Hill, 1935). pp. 210-221. The helium atom and exchange integrals.
L. Stryer, Biochemistry, 3rd ed. (New York: W. H. Freeman, 1988). Chapter 7. Myoglobin and hemoglobin.
(C) Turning into Iron:
Fossils do contain "Iron Pyrite":
From http://www.tmm.utexas.edu/npl/glossary.htm:
The mineral iron pyrite, FeS2, can occur in igneous, metamorphic and sedimentary rocks. It is often found in fossil shells, and can become a problem in museum collections because it may React with oxygen and water to form compounds that may destroy the specimen.
..............
One example of chemical reactions that may harm fossils is the changes that can occur in iron pyrite (FeS2). The sulfide component oxidizes to FeSO4 in the presence of oxygen, which leads to the production of sulfuric acid (H2 SO4) if water (H2O) is available. A simplified reaction is shown below:
4FeS2 + 13O2 + 2H2O -> 4FeSO4 + 2H2 SO4+ 2SO2
The reaction is more commonly observed where the pyrite occurs in microcrystals. The resulting 'decay' is marked by yellow-white efflorescences, cracking of the specimen, sulfurous odor, and scorched boxes or labels where they come in contact with the reacting specimen.
From http://www.g6csy.net/fossil/misc.html:
"(Iron) Pyrite is easily formed by decomposing organic matter when in the presence of iron."
"Sometimes the pyrite forms on the clay and shale to produces 'nodules of gold'....
Depending on how 'fresh' the (Iron) pyrite fossils are, they can range from either a dull-brown to a shiny-gold in colour. Unless properly treated after they have been collected, they will eventually corrode into a pile of rust, (actually into a mineral known as Melanterite, with the chemical formula FeSO4.7H2O).
The (Iron) pyritised fossils should be soaked in water to remove any salt or acid, dried slowly and then coated in polyethylene glycol (PEG), or varnish, in order to preserve them. In older days, shellac dissolved in alcohol, was used."
From http://www.cr.nps.gov/museum/publications/conserveogram/11-02.pdf:
Pyrite decay
Some geological specimens are susceptible to “pyrite decay. ” The iron pyrite in these specimens can react with water and oxygen in the atmosphere to convert to iron sulfate. This compound is greater in volume than iron pyrite and typically causes the specimen to crack into pieces and fall apart. Acid produced in the reaction also helps to destroy the specimen. Typically, the end result of pyrite decay will be the reduction of the specimen to a pile of yellow/gray powder. Pyrite decay can be triggered by RH over 60%.
The pdf file is also stored on my site, incase their site is down or the article disappears.
From http://www.arches.uga.edu/~ting/6200/project/encyclopedia1.htm:
Obsidian is a type of dark rock, which looks like glass. Obsidian may be black,
dark gray, red, or brown, with the color depending on the presence of tiny particles of iron minerals. (Physical geology, 1994,
p58) It is typically very sharp on its edges, and was used by ancient cultures in the
manufacture of knives, arrows and spear points. It is
composed mostly of SiO2, silicon dioxide, the main component of sand.
Volcanic ash is composed of very small shards of this material.

Picture: From http://library.thinkquest.org/J0110482/
From http://images.google.com/images?hl=en&lr=&q=iron+pyrite&btnG=Search:
Let us look at some sample pictures of Iron Pyrite:


3- According to Quantum Mechanics, all matter (including humans) will turn into iron:
From http://www.universalunity.net/iron2.htm:
This Muslim site doesn't have references, but the sites below do contain the references and they do confirm its claims:
We can summarize these developments by stating that matter continues to seek its lowest energy level, most stable form ( it achieves it through decay and subatomic particle exchange). We know that iron is the most stable element in the Universe, According to the rules of Quantum Mechanics , given enough time , all matter ( stars, trees, planets, humans, etc.,) will eventually turn into iron. In fact some scientists have done speculative calculations showing that in about 10^1435 years essentially all that is left will turn into " purest iron, the element possessing the most stable nucleus. " Then over even a longer span, in about 10 ^10^76 years, more transformation will take place and all that is left according to these calculations will be: "wandering bits of iron, few stray photons and black holes, " : even if you turn into rocks or iron..... or any creation which, in your minds , appears even more difficult , you will be resurrected......"
From http://www.nationmaster.com/encyclopedia/Iron:
Encyclopedia: Iron
.......
The nucleus of iron has the highest binding energy per nucleon, so it is the heaviest
element that is produced exothermically through fusion and the lightest through fission.
When a star that has sufficient mass to produce iron does so, it can no longer produce
energy in its core and a supernova will ensue.
Cosmological models with an open universe predict that there will be a phase where as a
result of slow fusion and fission reactions, everything
will become iron.
.......
See also set of irons.
nds:Isen sv:J rn zh-cn:? Category:Chemical elements Category:Metals
From http://www.campusprogram.com/reference/en/wikipedia/i/ir/iron.html:
Reference Library: Encyclopedia
Iron
.......
The nucleus of iron has the highest binding energy per nucleon, so it is the heaviest element that is produced exothermically through fusion and the lightest through fission. When a star that has sufficient mass to produce iron does so, it can no longer produce energy in its core and a supernova will ensue.
Iron is the most common metal in the universe.
Cosmological models with an open universe predict that there will be a phase where as a result of slow fusion and fission reactions, everything will become iron.
From http://www.free-definition.com/Iron.html:
The nucleus of iron has the highest binding energy per nucleon, so it is the heaviest element that is produced exothermically through fusion and the lightest through fission. When a star that has sufficient mass to produce iron does so, it can no longer produce energy in its core and a supernova will ensue.
Cosmological models with an open universe predict that there will be a phase where as a result of slow fusion and fission reactions, everything will become iron.
Let's look briefly at what the field of Quantum
Mechanics is for general knowledge:
From http://franklaughter.tripod.com/cgi-bin/histprof/misc/atomic.html:
Atomic Theory
Atom is one of the basic units of matter
Atom is one of the basic units of matter. Everything around us is made up of atoms. An atom is incredibly tiny--more than a million times smaller than the thickness of a human hair. The smallest speck that can be seen under an ordinary microscope contains more than 10 billion atoms. The diameter of an atom ranges from about 0.1 to 0.5 nanometer. A nanometer is a billionth of a meter, or about 1/25,000,000 inch.
Atoms form the building blocks of the simplest substances, the chemical elements. Familiar elements include hydrogen, oxygen, iron, and lead. Each element consists of one basic kind of atom. Compounds are more complex substances made of two or more kinds of atoms linked in units called molecules. Water, for example, is a compound in which each molecule consists of two atoms of hydrogen linked to one atom of oxygen.
..............
The forces within an atom
The field of physics called quantum mechanics deals with the forces inside an atom and the motions of subatomic particles. This field began in 1913, when the Danish physicist Niels Bohr used the quantum theory to explain the motion of electrons in atoms.
Electron energy levels. According to quantum mechanics, electrons cannot have just any amount of energy. Instead, electrons are restricted to a limited set of motions, each of which has a specific value of energy. These motions are called quantum states or energy levels. When an electron is in a given quantum state, it does not absorb or give off energy. For this reason, an atom can gain or lose energy only if one or more electrons change their quantum state.
Just as water always seeks its lowest possible level, electrons seek the state of lowest energy. However, only one electron at a time can occupy each quantum state. If the lower states are filled, other electrons are forced to occupy higher states. If all electrons are in the lowest possible state, the atom is in its ground state. This condition is normal for atoms at ordinary temperatures.
When matter is heated to temperatures higher than a few hundred degrees, energy is available to raise one or more electrons to a higher energy level. The atom is then in an excited state. However, atoms rarely remain in an excited state for more than a fraction of a second. An excited electron almost immediately drops to a lower state and continues dropping until the atom returns to its ground state. At each succeeding drop, the electron gives off a tiny packet of radiant energy called a photon. The energy of the photon equals the difference between the two energy levels of the electron. The photons given off by electrons are detected as visible light and other forms of electromagnetic radiation.
Bohr originally described the quantum states of electrons as orbits like those of the planets around the sun. However, physicists now know that this description is incorrect because an electron is not simply a particle. An electron also has some characteristics of a wave. It is difficult to imagine how something could be both a particle and a wave. This difficulty is one of the problems scientists have in trying to describe the atom to nonscientists. To do so, scientists must use familiar ideas based on our knowledge of the world as we observe it. But conditions inside the tiny atom differ greatly from those in our everyday world. For this reason, physicists can describe the motions of electrons accurately and completely only in mathematical terms.
Forces in the nucleus. The quantum rules that govern the motions of electrons also apply to the motions of protons and neutrons inside the nucleus. However, the force that keeps the nuclear particles together differs greatly from the electrical attraction that holds the electrons within the atom. Each nuclear particle is attracted to its nearest neighbor by what is called the nuclear force or, sometimes, the strong interaction. Like electric charges repel each other. However, the powerful nuclear force overcomes the mutual repulsion of the positively charged protons. It thus keeps the nucleus from flying apart. This force dies off quickly, however, unless the nuclear particles are extremely close together. Electrons are immune to the nuclear force.
The nuclear force is highly complicated, and no exact mathematical description of it has been formulated. Nevertheless, a theory known as the nuclear shell model provides reasonably accurate estimates of the energy levels in the nucleus.
One neutron and one proton can occupy each quantum state in the nucleus. For this reason, a light nucleus has a nearly equal number of protons and neutrons. But a proton and a neutron in the same state do not have the same amount of energy. Each proton is electrically repelled by all other protons in the nucleus, which increases its energy. In a nucleus with many protons, the difference in energy levels between protons and neutrons is considerable, and more low-energy states are available for neutrons than for protons. This is why a heavy nucleus has more neutrons than protons.
..............
Contributor: Robert H. March, Ph.D., Prof. of Physics and Integrated Liberal Studies, Univ. of Wisconsin, Madison.
Additional resources
Level I
Berger, Melvin. Our Atomic World. Watts, 1989.
Bronowski, J., and Selsam, M. E. Biography of an Atom. 1965. Reprint. Harper, 1987.
Cooper, Chris. Matter. Dorling Kindersley, 1992.
Lampton, Christopher F. Particle Physics. Enslow, 1991.
Newton, David E. Particle Accelerators: From the Cyclotron to the Superconducting Super Collider. Watts, 1991.
Level II
Asimov, Isaac. Atom: Journey Across the Subatomic Cosmos. Dutton, 1991.
Glashow, Sheldon L. The Charm of Physics. Am. Inst. of Physics, 1991.
Lederman, Leon M., and Schramm, D. N. From Quarks to the Cosmos: Tools of Discovery. Scientific Am. Bks., 1989.
Lederman, Leon M., and Teresi, Dick. The God Particle: If the Universe Is the Answer, What Is the Question? Houghton, 1993.
Von Baeyer, Hans C. Taming the Atom: The Emergence of the Visible Microworld. Random Hse., 1992.
SOURCE: IBM 1999 WORLD BOOK
4- Conclusion:
The Noble Quran was perfectly accurate about humans turning into fossils and iron. As I proved above, the Divine Claim in the Noble Quran was Scientifically proven to be true. Prophet Muhammad peace be upon him, who lived in the desert 1500 years ago, could not have known this, nor was this information known to man back then.
The Noble Quran has overwhelming amount of Scientific Claims and Miracles in it that prove Its Divinity and Truthfulness. Embrace Islam and you will be saved.
Back to Science in the Noble Quran and Islam.
The amazing creation of earth and iron in the Noble Quran. Iron came from space, and the Noble Quran mentioned it.
The 7 properties of earth in the Noble Quran and Science. Read the first section of the article.
Rebuttal to the "Heaven" and "Stars in the lower Heaven" in Noble Verses 37:6 and 65:12. Read the "Rebuttals" section in the article. This was an attempt to refute the fact that Allah Almighty Claimed that He Created 7 Ozone Layers as well as 7 different Heavens.
Living Creatures were sent down from space. Science confirms the Noble Quran's Claim.
Allah Almighty said that the earth is "egg-shaped".
The Earth is round according to Islam.
The amazing creation of earth and mountains in the Noble Quran.
Geology in the Noble Quran - See the Scientific confirmation.
Oceanology in the Noble Quran - See the Scientific confirmation.
http://www.universalunity.net/iron.htm This article shows the Mathematical Codes of Iron in the Noble Quran and Science, and shows how Science confirmed Allah Almighty's Divine Claim in Noble Verses 17:49-50 about the dead converting into rocks and iron. In case the web site is down, you can access the article on my site.
http://www.universalunity.net/iron2.htm More elaborations on Noble Verses 17:49-51. In case the web site is down, you can access the article on my site.
AUDIOs from Dr. Zaghlool Al-Najjar
Lack of Oxygen and low air pressure in space.
The constant reduction of earth's size: The earth used to be 200 times bigger!
Iron was sent down to earth from space.
The origins of the Universe, Big Bang theory, Cosmic Crunch, and the creation of the SECOND EARTH after the second Big Bang in the Noble Quran and Science. Also, Dr. Zaghlool's scientific explanation of the earth and heaven coming to Allah Almighty "willingly or unwillingly" and how each celestial object behaves differently depending on its mass.
The Cosmic Crunch and the creation of the second Universe and second earth.
Time and the Speed of Light precisely calculated and mentioned in the Noble Quran.
Mary: Fornicate with you "in my mother's bed"
200 years no gospels | 24 other thrones besides Jesus' | Jesus was created | Isaiah 53 mistranslations | What's new | A-Z library (3300+ articles) | "Muslims" was the original title | Quran Search | Quran Moral Code (100s of them) | Quran: Bibles are mostly of corrupt قول | Research & Blog | 9/11 Israel-lie | Youtube
Quran's STUNNING Divine Miracles: [1] Allah Almighty also promised in several Divine Prophecies that He will show the Glorious Quran's Miracles to mankind. For example:
Coincidence? See 1,000s of examples [1].Quran's Stunning Numerical & Scientific Miracles.
"I cannot do anything on my own." (i.e., Jesus could not perform a single Miracle without GOD sending it down to him first!). I can not perform a single Miracle on my own!! I am totally POWERLESS without Allah Almighty! John 5:31 "if I (Jesus) bear witness of myself, then I would be a liar!" GOD didn't talk this way when He spoke to Moses. GOD's testimony alone is always sufficient! Jesus also bowed his face down to the ground, like we Muslims (Isaiah 56:5: Muslim is the future believers' name, and sons and daughters of GOD titles will be "no more"; |